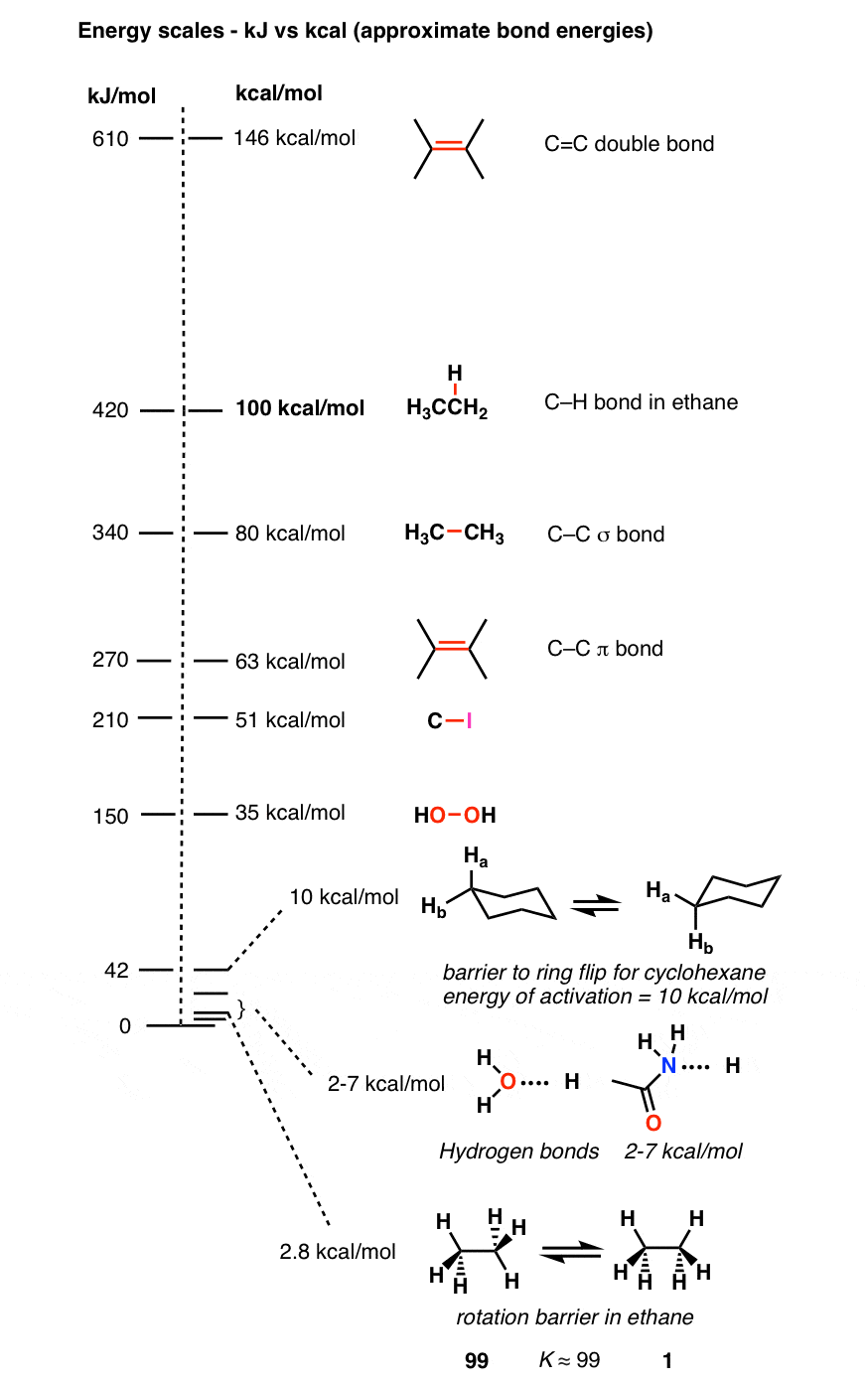
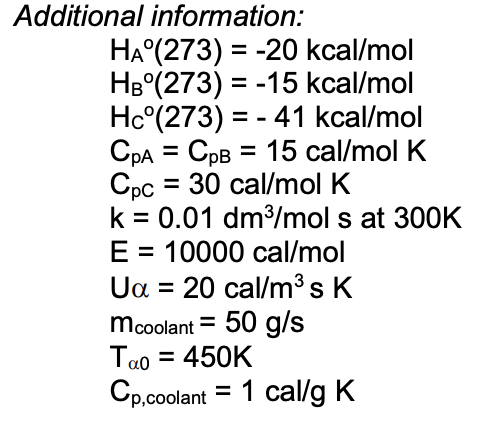
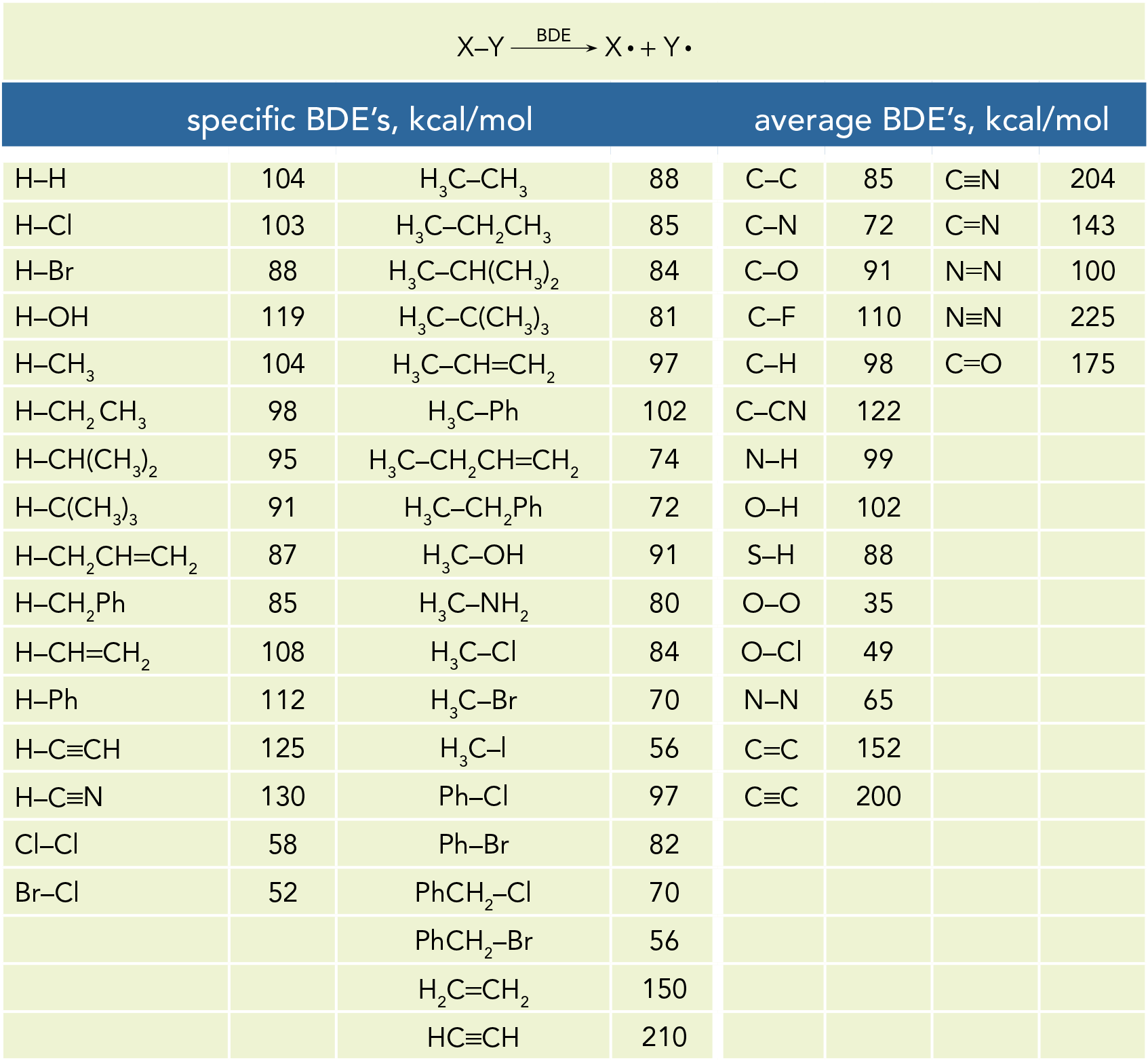
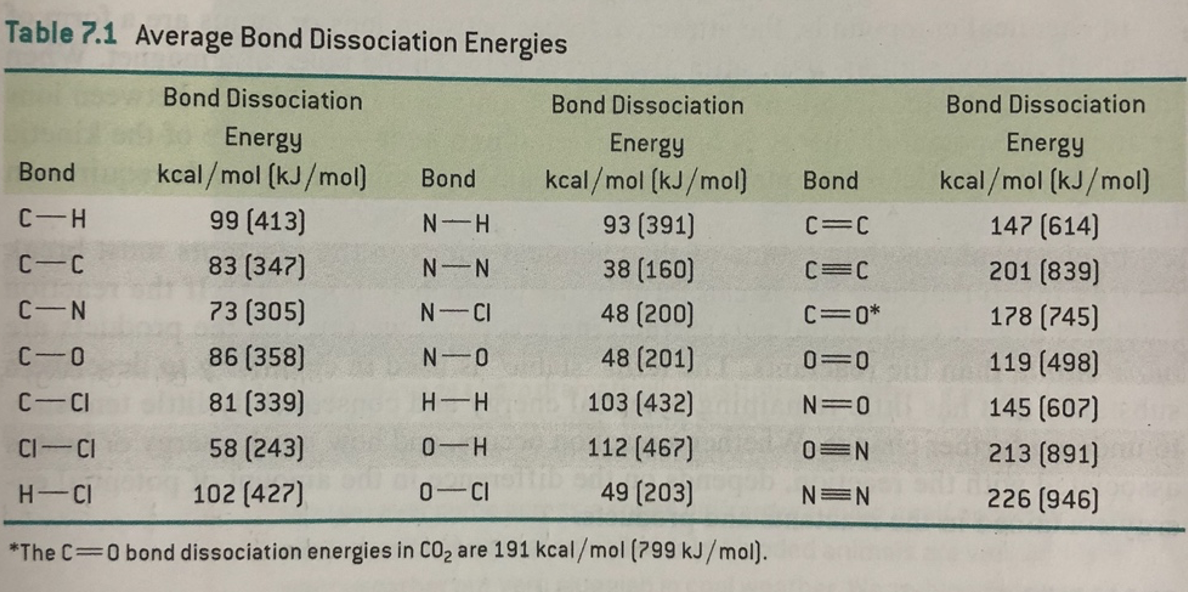
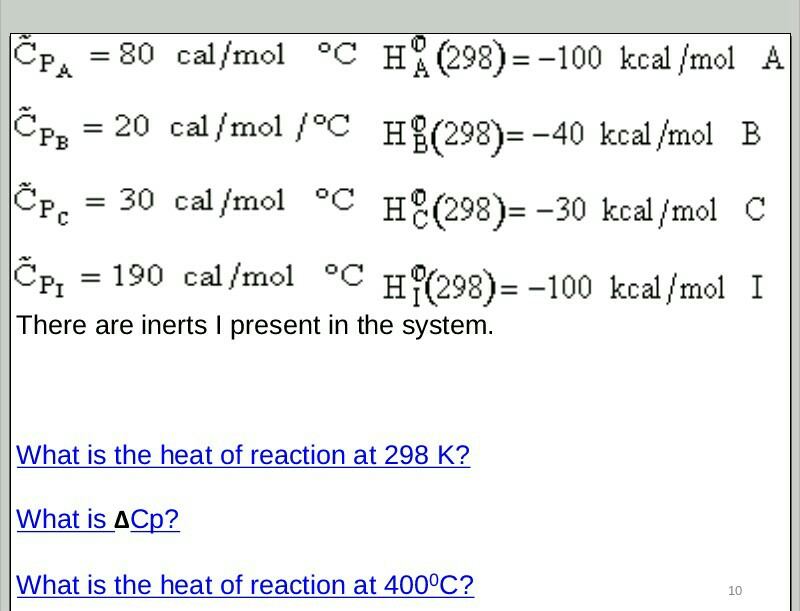
Forces Note on units: Energy kcal/mol(1kcal = kJ). DistanceÅ(1Å = M) At room temperature thermal energy = RT=.59 kcal/mol I. Covalent bonds. - ppt download

IJMS | Free Full-Text | Density Functional Theory Study of Low-Dimensional (2D, 1D, 0D) Boron Nitride Nanomaterials Catalyzing Acetylene Acetate Reaction

Standard heat of formation of KI is - 78.31 kcal mol^-1 . Calculate its lattice energy from following information: IE (K) = 4.3 eV EA (I) = - 73.4 kcal mol^-1 Bond
The lattice enthalpy of KI will be, if the enthalpy of (I) Δ_fH^°(KI) = –78.0 kcal mol^ 1 (II) Ionisation energy of K to K+ is 4.0 eV (III) Dissociation energy of

Forces Note on units: Energy kcal/mol(1kcal = kJ). DistanceÅ(1Å = M) At room temperature thermal energy = RT=.59 kcal/mol I. Covalent bonds. - ppt download
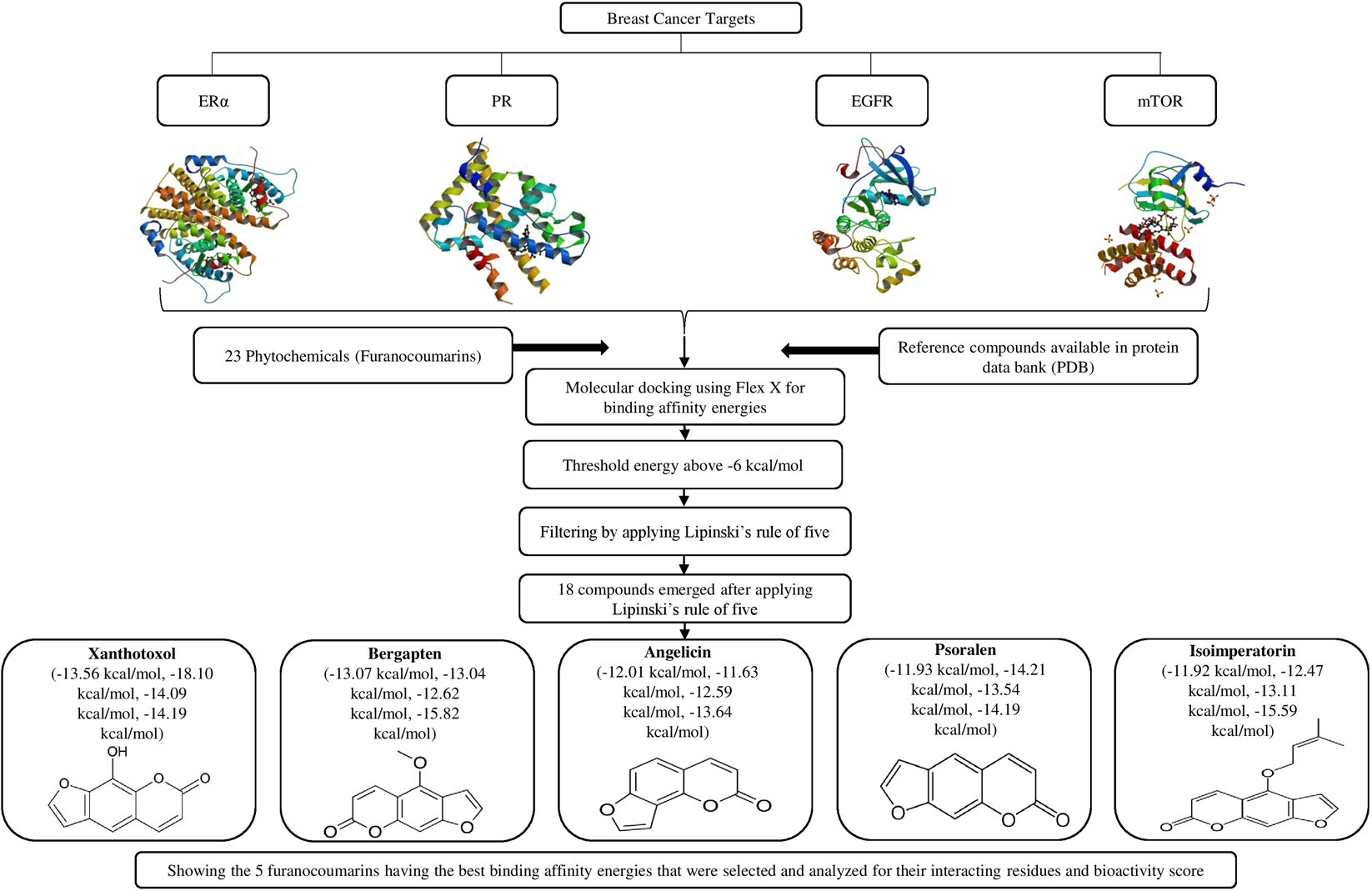
Structure Based Multitargeted Molecular Docking Analysis of Selected Furanocoumarins against Breast Cancer | Scientific Reports

From the given table answer the following questions :CO(g) CO2(g) H2O(g) H2(g) ( Δ H^∘ f )298 ( - kCal / mole ) - 26.42 - 94.05 - 57.80 ( Δ G^∘
En = –313.6/n2 kcal/mole. If the value of E = –34.84 kcal/mole, to which value does 'n' correspond? (1) 4 (2) 3 (3) 2 (4) 1